Overcoming challenges to advance new business models in pharma
Simone Rebora • 24 marzo 2021
Despite all the challenges and pipeline competition, to date new therapies has shown unprecedented efficacy: for instance, consider the impact for the patients. As a result, new therapies with new models will likely remain an important treatment option for end-stage tumor, as well as Covid and SARS vaccines.

Despite the arrival of new exciting therapies, several advances are still needed to expand its application into earlier lines of therapy. Therefore, supply chain and business quality must invest in critical gaps and barriers, expanding treatment models into new healthcare settings, reducing the innovation cycle time to enable success in all the new contexts and transforming manufacturing processes. An interesting Mckinsey article examined some useful points that I share with you.
Optimization of autologous CAR Ts for liquid tumors
Despite all the challenges and pipeline competition (such as from bispecifics), to date new therapies has shown unprecedented efficacy: for instance, consider the impact for the patients, they can have a great hope who patients never had before. As a result, CAR T will likely remain an important treatment option for end-stage-liquid-tumor patients, as well as the Covid and SARS vaccines.
Which kind of technologies and processes will further improve the process, mitigate challenges, and raise the tide for the entire industry:
Advanced gene transfer tools can improve efficiency. The industry faces a well-known viral-vector capacity constraint and a limited number of third-party suppliers, so manufacturers must either invest heavily to build in-house production or lock down contracts with these viral-vector manufacturers.¹² Using advanced gene-transfer tools may also allow testing multiple modifications in a modular fashion, reducing innovation cycle time through faster, less expensive testing.
Innovate the patient journey to collect healthier patient cells earlier in the treatment cycle.
Currently, relapsed or refractory patients—those who have exhausted more conventional therapies—demonstrate inconsistent apheresis cell recovery due to multiple rounds of lymphocyte-depleting therapies, such as chemotherapy, prior to CAR T treatment. Collecting and storing healthy lymphocytes from patients earlier in their treatment cycle may therefore serve as a sort of insurance policy for patients who eventually progress to relapsed or refractory stages and need CAR T treatments. If redundant transportation is cut out, healthier and ready-to-use cells for expansion could also reduce vein-to-vein time by about a week. However, today’s storage infrastructure is not in place to ensure the collection and long-term storage of large numbers of patient samples. Given that most earlier-line therapies for liquid tumors are not curative, many more stored samples will be used than were previously, thus raising the bar for storage quality.
Implement harmonized procedures to remove apheresis-capacity constraints. As more candidates enter clinical trials and CAR T achieves commercial success, the apheresis-center capacity bottleneck will remain. This burden is exacerbated by the significant time and resources required to open each apheresis facility—including required training, compliance or legal documentation, and audits, all of which may vary for each company.¹⁴ A centralized organization that standardizes apheresis or manages capacity and distribution among apheresis centers working with manufacturers can streamline procedures, freeing up valuable time and resources to serve patients.
Enable fast turnaround and quality assays.
Many simple quality-control assays (regarding sterility or mycoplasma, for example) add significant uncertainty and length—on average a few days, but sometimes up to weeks—to the current process. Some manufacturers have no choice but to insource these routine tests that have significant economies of scale to reduce vein-to-vein time. High-quality assays, process innovations (such as cloud-based data transfer), and tracking and validating each step of manufacturing all help reduce turnaround time.
Adoption of traditional healthcare settings to administer CAR Ts
Nearly all CAR T treatments are provided in inpatient settings at AMCs. While this highly concentrated expertise allows for close monitoring of adverse events, it also is extremely cumbersome for patients who often need to travel multiple times (for diagnosis, apheresis, or transfusion) while with an advanced-stage cancer. If appropriate training, quality collection and administering of therapy, and patient safety are properly ensured, then adoption of the following traditional healthcare settings could significantly improve patient pain points.
Outpatient settings.
Compared with inpatient reimbursement, treatment in outpatient facilities can increase number of patients who would benefit from CAR T and improve economics for hospitals. The main challenge will be ensuring closely monitored and addressed patient safety—for example, through tech-enabled monitoring or sufficient training.
Community transplant centers. Community transplant centers provide access to a more diverse patient population, which becomes more important as more CAR T therapies are developed. Because of the low referral rates from the community setting to AMCs (such as for multiple myeloma), expanding channels to community transplant centers could significantly increase the volume of patients who could benefit from CAR T.
Considering the vaccines, in addition to the logistics challenges that have slowed the rollout of the highly effective Pfizer-BioNTech and Moderna Covid-19 vaccines, there is another obstacle that needs to be overcome: the large numbers of people — up to 40% of the U.S. population — who say they won’t get vaccinated. Then what we should do?
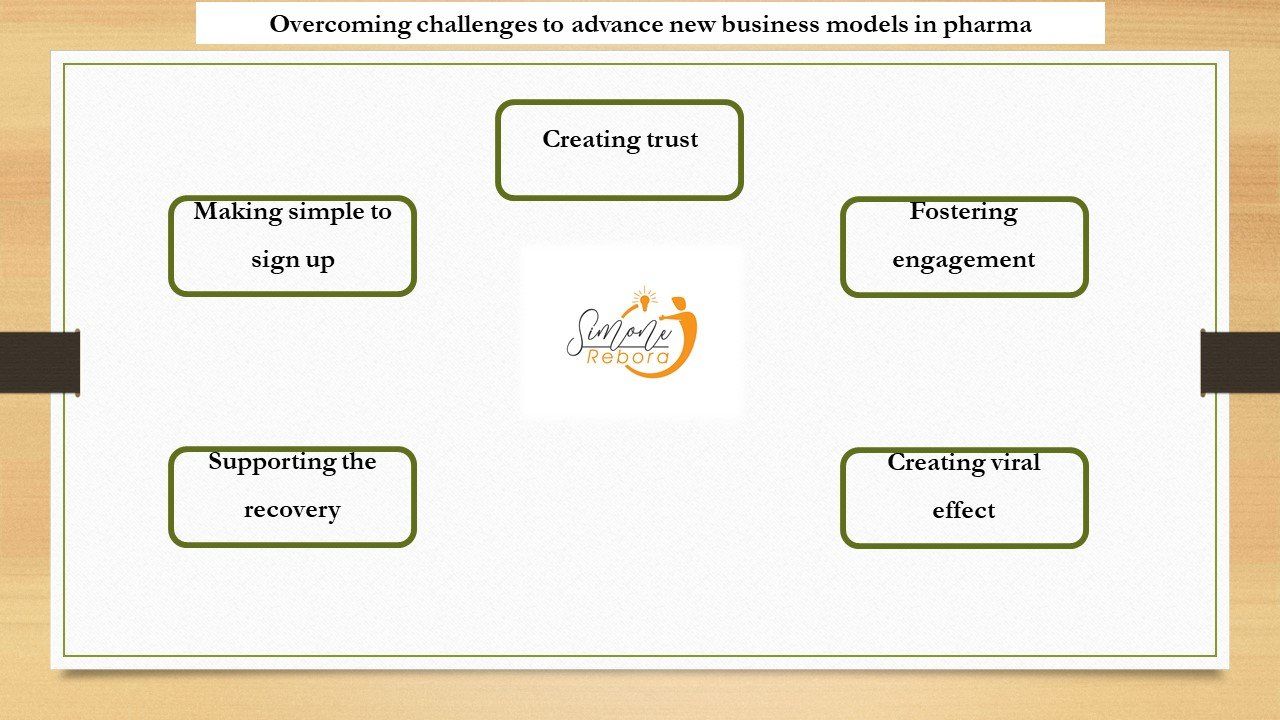
- Creating trust. Much of the hesitation to get a Covid-19 vaccine stems from the lack of trust in the health care system, the pharmaceutical companies that brought the vaccines to market in record time, in some vaccination advocates
-
Making it simple to sign up. Often the sheer complexity of the health care system prevents people from getting the right care. Once there is interest in getting the vaccine, people need to know when and where to get it
- Acing the vaccination. Getting a shot isn’t pleasant, but there is plenty we can do to make it a good experience. We have the potential to help people feel as comfortable as possible and to be proud of their contribution to keeping us all safe.
- Supporting the recovery.
The hardest part of the vaccination process is actually the one to three days after someone has received the shot when a significant proportion of people experience side effects, which range from pain at the injection site to headaches to low-grade fevers.
- Fostering engagement. Unfortunately, getting the shot doesn’t mean people can immediately go back to their pre-2020 ways of living. Immunity to the vaccine takes days to build, a second dose of the vaccine is vital, and we still need to wear masks after getting vaccinated. Herd immunity depends on people understanding this.
- Creating viral effects.
Diffusion of innovation is ultimately a social process. As more and more people take the vaccine, getting the last cohort of people who are most resistant to taking the vaccine, the “laggards,” will be a challenge. In addition to the tactics mentioned above to build trust and celebrate people who get the shot, we can borrow from efforts used to get Americans to vote: e.g., “I voted” Facebook picture frames, the use of celebrity influencers, and direct outreach by nonprofit organizations
By thinking of the vaccine as a consumer product, vaccination as a service, and a high NPS as a goal, we can better design the end-to-end vaccination process and bring this pandemic to a close as quickly as possible.

Over the past months, through my LinkedIn series “Chapter: The Promise Journey,” I have explored how AI is weaving a transformative red thread throughout pharma’s complex ecosystem. This article offers a comprehensive summary and reflections on the early stages of this journey, highlighting the immense promise AI holds, and the leadership and cultural shifts required to fully realize its potential.

As artificial intelligence reshapes the pharmaceutical landscape, I am compelled to synthesize the key milestones and reflections from my LinkedIn series, The Promise AI Journey, into this first comprehensive summary. This article distills lessons from building AI roadmaps, navigating Pharma’s unique challenges, and envisioning a future where AI accelerates both innovation and ethical responsibility.